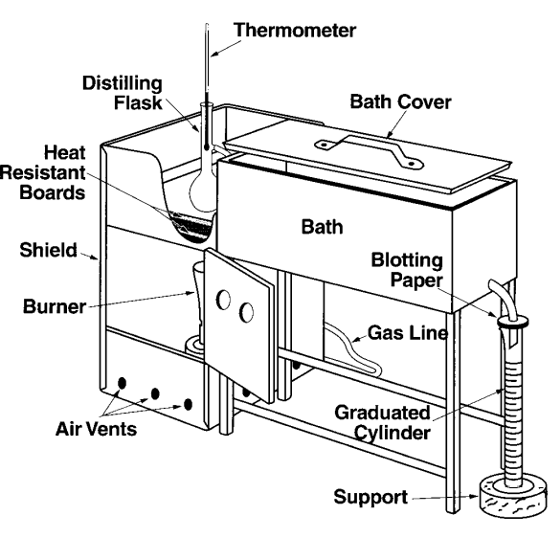
ASTM D-86 Distillation
D-86 is not a true distillation because there is only one stage. Consequently, the D-86 tends to have a much higher IBP temperature than the actual on due to entrainment as molecular interactions hold lighter molecules in the mixture. At the tail end of the D-86 distillation, due to entrainment, the heavier molecules can flash off readily, resulting in a lower EP (end-point) than the actual distillation. Therefore, D-86 has a higher IBP and a lower EP than the actual atmospheric distillation in a refinery. However, the deviations in the front and tail ends in D-86 distillation can be overcome by TBP distillation. It is important to know that the D-86 distillation can be correlated with TBP distillation.
Commercial Yields Versus Theoretical Yields
It is common that commercial yields from a refiner cannot match the theoretic yields obtained form TBP distillation in a crude assay because commercial distillation is not perfect with lighter materials slumping into heavier cuts. Typically, there are 6-10 fractionation theoretical stages between two adjacent cuts in a commercial crude distillation column in comparison with very large number of fractionation stages assumed in the TBP distillation. Not only are the product yields different but also the product properties can vary. A useful tool to verify the information in a crude assay is called crude oil breakup.
A crude breakup is performed to predict the potential gap or overlaps in product yields between crude assay and operation based on actual process conditions including flash zone temperature and pressure. The predicted product yields and properties can be used to compare with that claimed in crude assay and more importantly as guidelines for refinery operations, for example, determining distillation cuts and predicting their properties.
'Physical properties' 카테고리의 다른 글
| True boiling point and ASTM D86 (0) | 2017.02.23 |
|---|---|
| 농도 환산 어떻게 계산해야 할까요? (0) | 2016.04.26 |
| 농도 계산은 어떻게 할까요? / Concentration calculation (0) | 2016.04.26 |
| 노르말 부피는 어떻게 구할까요? / Nm3 (0) | 2016.04.25 |
| K factor vs. UOP K factor (0) | 2016.04.03 |